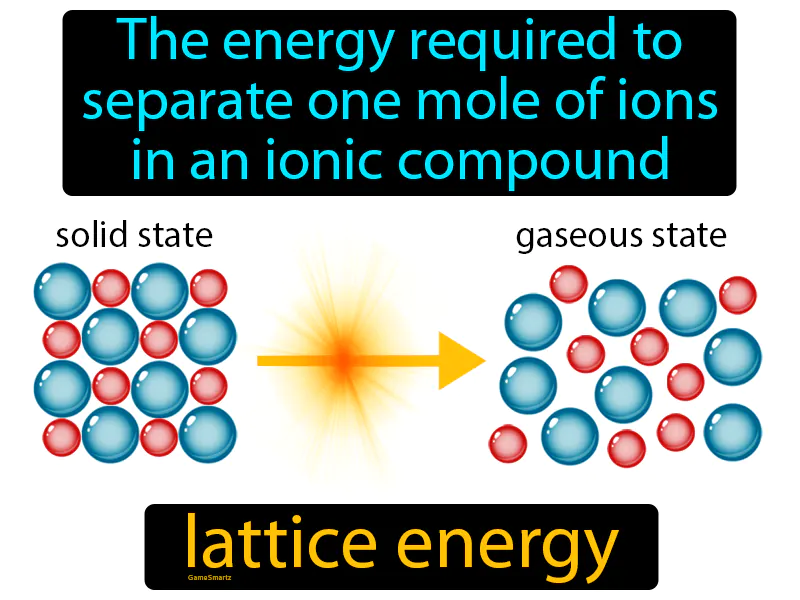
Lattice Energy
Lattice Energy: Easy to understand
Imagine trying to untangle a pair of tightly knotted earbud wires. Just like the effort required to carefully and methodically separate each twist and knot, lattice energy is the energy needed to pull apart the tightly bound ions in an ionic compound. In this analogy, the earbud wires represent the ions, the knots symbolize the electrostatic forces holding them together, and the effort to untangle them mirrors the energy needed to separate those ions.
Practice Version
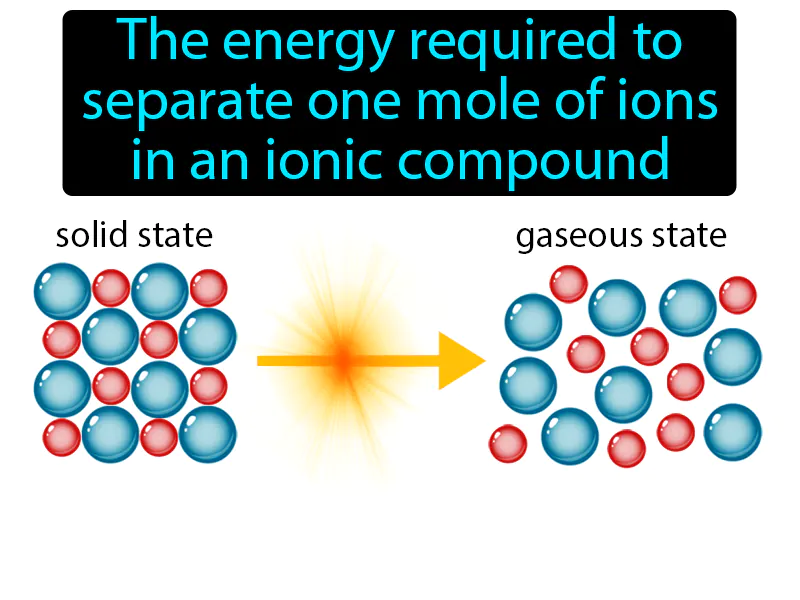
Lattice Energy: The energy required to separate one mole of ions in an ionic compound. Lattice energy. Lattice energy is the energy needed to break the bonds between ions in a solid ionic compound.