Electron Sea Model
Electron Sea Model: Easy to understand
Imagine trying to move through a crowded dance floor where everyone is swaying to the music, yet you can still weave through without much resistance. This is similar to how the electron sea model describes the behavior of electrons in a metal, where valence electrons move freely and fluidly around fixed positive metal ions. Just as the dancers on the floor can shift and adjust to allow someone to pass through, the electrons in the "sea" can move and redistribute themselves, allowing metals to be malleable and conductive without breaking their structure.
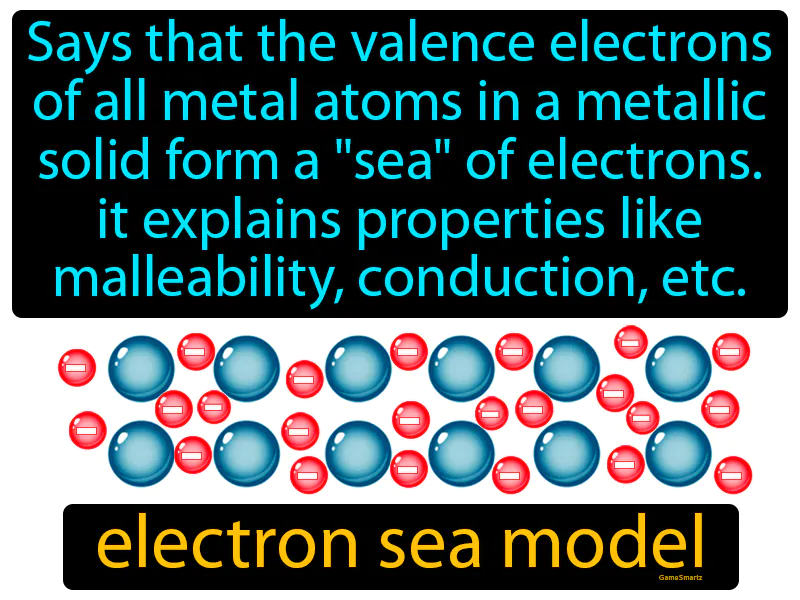
Practice Version
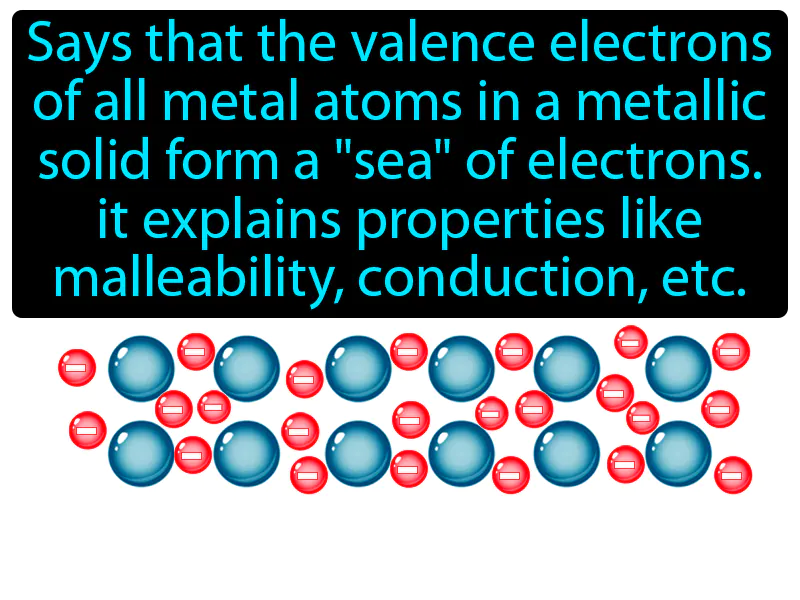
Electron Sea Model: "Says that the valence electrons of all metal atoms in a metallic solid form a 'sea' of electrons. It explains properties like malleability, conduction, etc., electron sea model." The electron sea model describes how electrons flow freely around metal atoms, allowing them to conduct electricity and be shaped easily.