Isotope
What is an Isotope? Explained in an easy to understand way:

What's Covered in the Video:
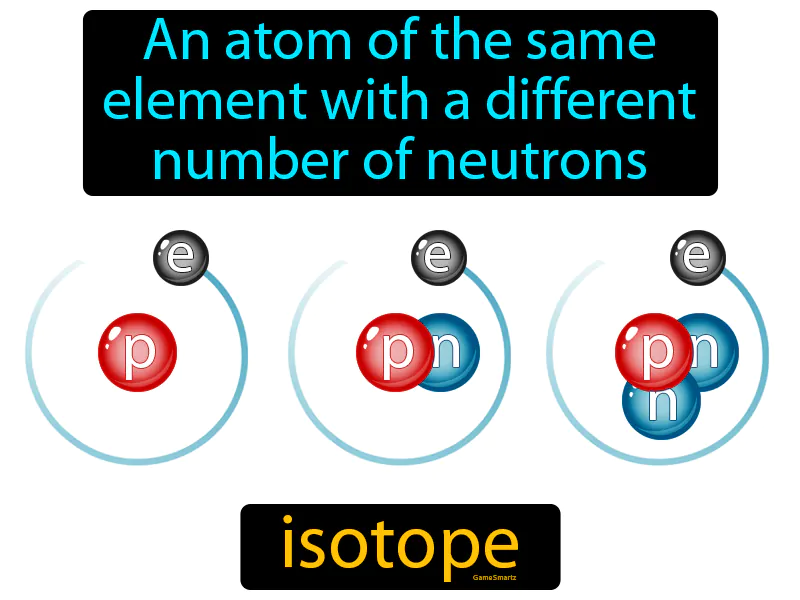
Imagine you're trying to organize a group of friends for dinner, but each friend has a slightly different schedule. This is similar to isotopes of an element, where each atom has the same type of protons (like your friends being from the same group) but varies in the number of neutrons (akin to their differing schedules). Just as your group remains the same despite each person having a unique availability, isotopes are atoms of the same element with different numbers of neutrons, maintaining their identity as the same element while differing slightly in composition.
Practice Version
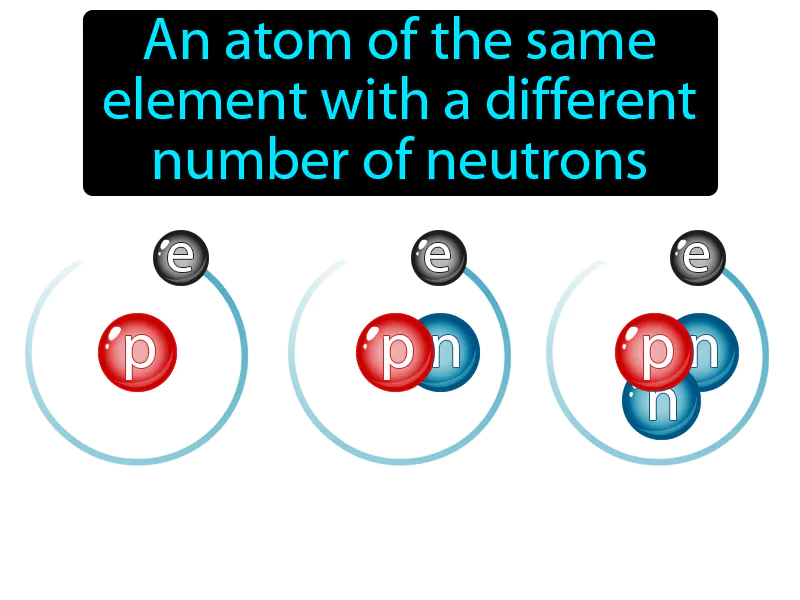
Isotope: An atom of the same element with a different number of neutrons. Isotope. Isotopes are variations of the same chemical element that have different numbers of neutrons in their nuclei.