Atomic Mass
What is Atomic Mass? Explained in an easy to understand way:

What's Covered in the Video:
Imagine trying to figure out the average cost of a grocery bill when sometimes you shop for a family gathering and other times just for yourself. This is similar to how scientists determine the atomic mass of an element, which is the average mass of all its isotopes, each with its own 'grocery bill' of mass depending on how frequently it 'shops' or occurs in nature. Just like you might spend more overall on groceries when you shop for a family gathering compared to just yourself, an element's atomic mass takes into account the 'spending' or contribution of each isotope based on how commonly it is found, much like calculating an average grocery cost over time.
Practice Version
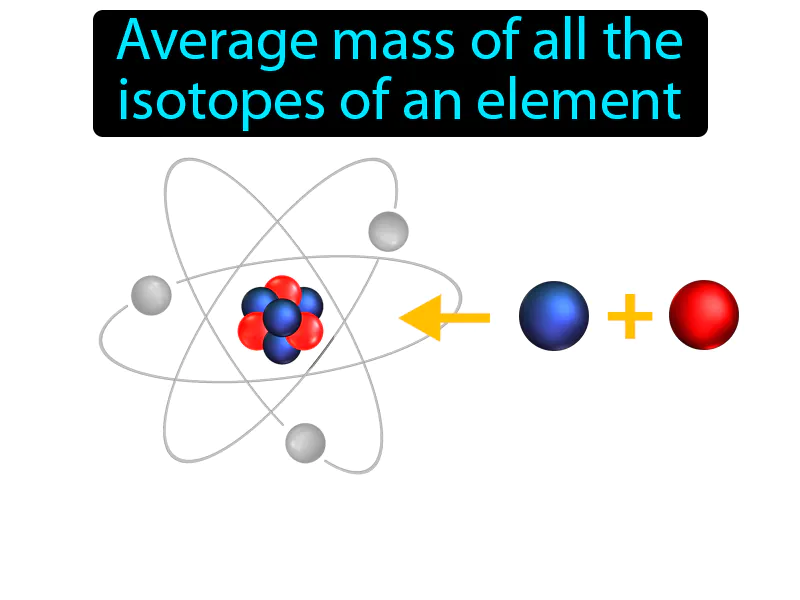
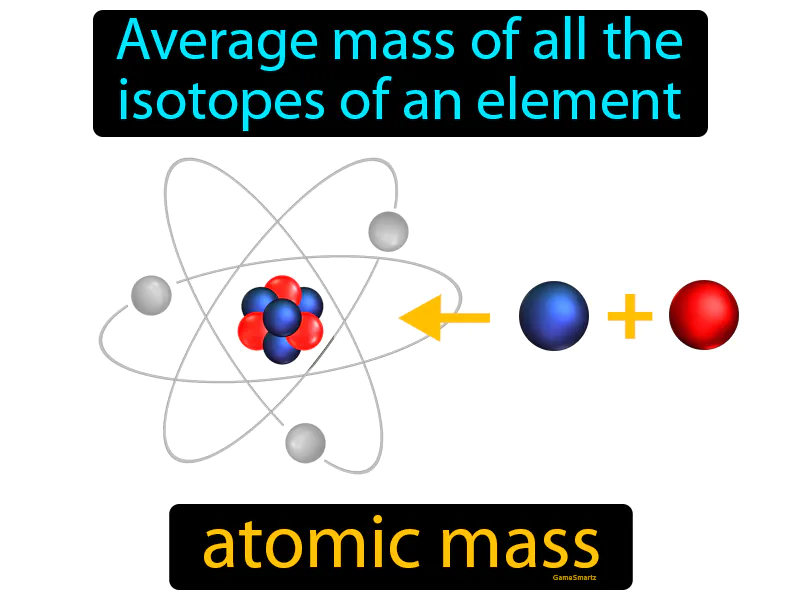
Atomic Mass: The average mass of all the isotopes of an element. Atomic mass. Atomic mass is the weighted average of the masses of all naturally occurring isotopes of an element.