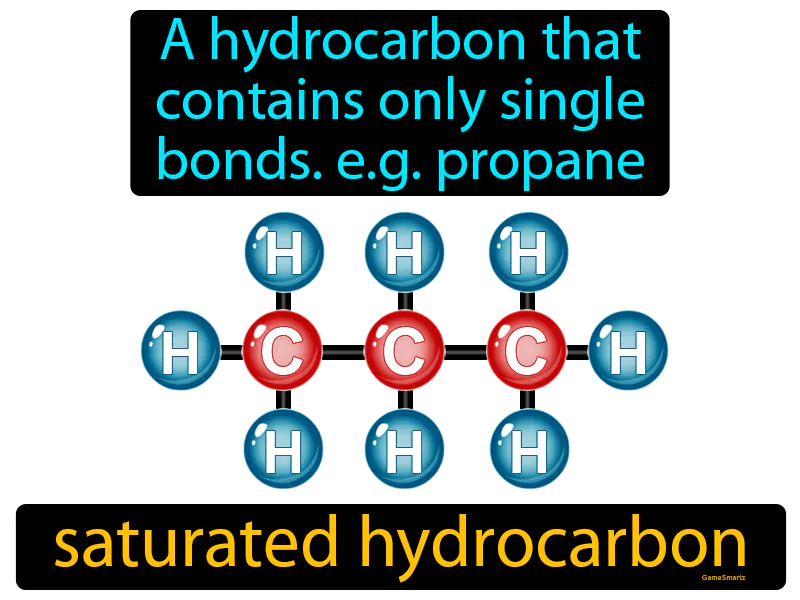
Saturated Hydrocarbon
What is a Saturated Hydrocarbon? Explained in an easy to understand way:

What's Covered in the Video:
Imagine trying to fit all your groceries into a single, sturdy shopping bag without any items poking through. Just like a shopping bag is filled to capacity with groceries and everything is securely contained, a saturated hydrocarbon, such as propane, is filled to its capacity with hydrogen atoms, with no room for additional bonds. The sturdy structure of the shopping bag, holding everything together tightly without any tears or open spaces, is analogous to the single bonds in propane that keep the carbon atoms fully saturated and stable.
Practice Version
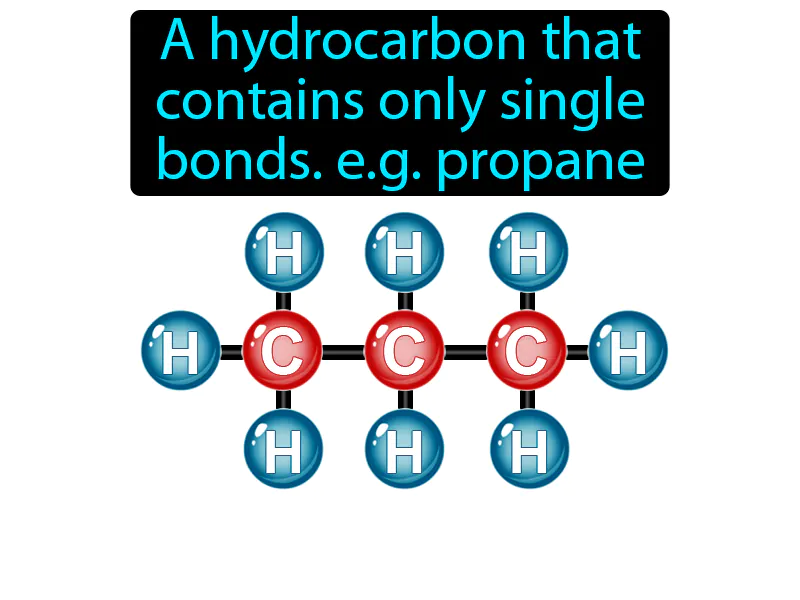
Saturated Hydrocarbon: A hydrocarbon that contains only single bonds, like propane. Saturated hydrocarbon. In simple terms, a saturated hydrocarbon is a molecule made of carbon and hydrogen atoms connected by single bonds, found in fuels like propane.