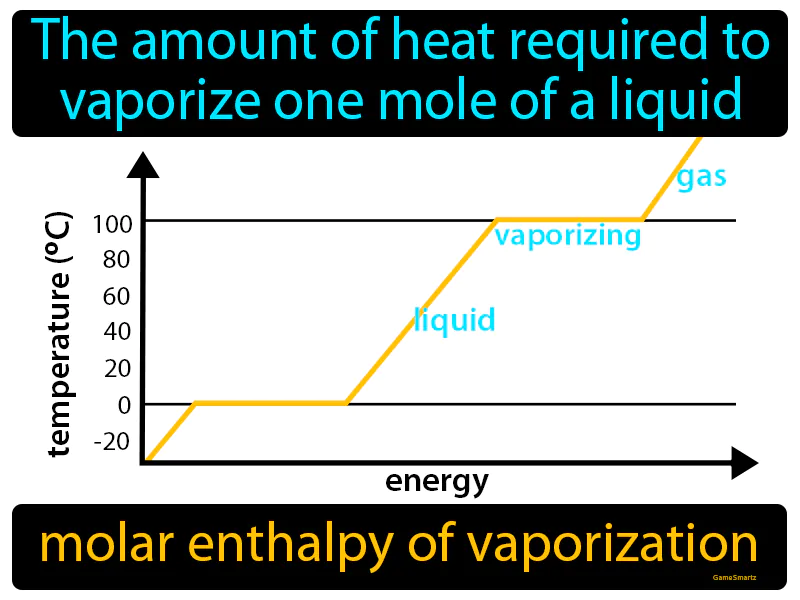
Molar Enthalpy Of Vaporization
Molar Enthalpy Of Vaporization: Easy to understand
Imagine trying to dry a soaked towel after a rainstorm. Just like the effort required to dry every bit of moisture from the towel, the molar enthalpy of vaporization is about providing enough heat to turn every molecule in a mole of liquid into vapor. Here, the wet towel represents the liquid, the effort to dry it symbolizes the energy or heat input, and the dry towel corresponds to the vaporized state, making the process of drying analogous to vaporization.
Practice Version
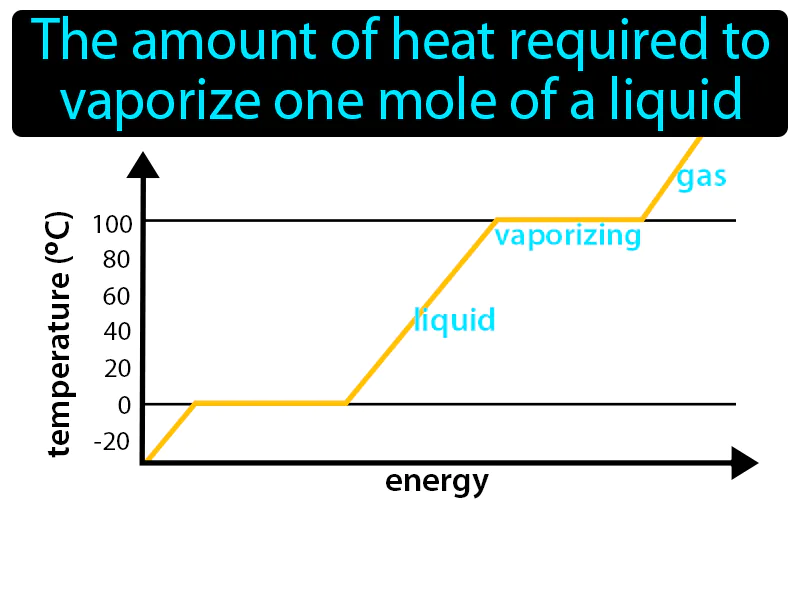
Molar Enthalpy Of Vaporization: The amount of heat required to vaporize one mole of a liquid is called the molar enthalpy of vaporization. It is the energy needed to turn a liquid into a gas at its boiling point.