Van Der Waals Force
What is Van der Waals Force? Explained in an easy to understand way:
What's Covered in the Video:
Imagine trying to stack a bunch of mismatched, slightly sticky playing cards into a neat pile, only to find they subtly cling to each other just enough to hold the stack together. This is similar to how Van der Waals forces work, where the weak attraction between the slightly charged regions of nearby molecules causes them to cling together. Just as the tackiness of the cards helps maintain the stack's structure without strong adhesive, the weak attractions in Van der Waals forces help stabilize molecular configurations by drawing them together through minute charges, even when they aren't strongly bonded.
Practice Version
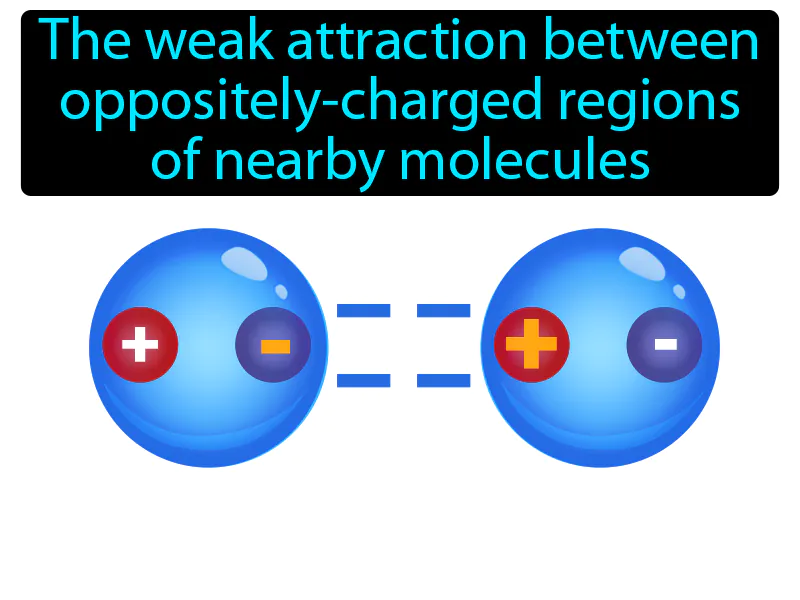
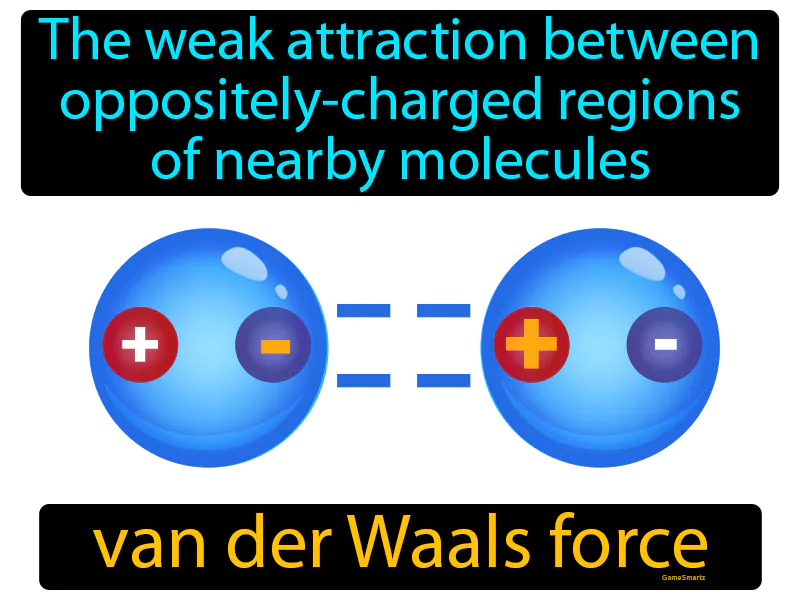
Van Der Waals Force: The weak attraction between oppositely charged regions of nearby molecules. Van der Waals force. It's a gentle force that makes molecules stick to each other because of tiny charges.