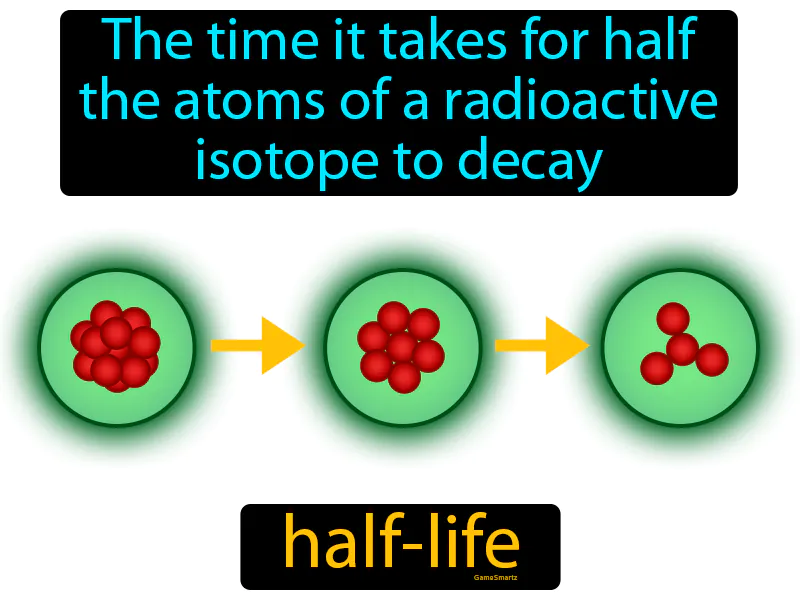
Half-life
What is Half-Life? Explained in an easy to understand way:

What's Covered in the Video:
Imagine you're trying to eat a giant chocolate cake all by yourself, but you can only eat half of what's left each day. Just like how it takes you progressively longer to finish the cake because you keep halving your daily consumption, a radioactive isotope takes a specific amount of time to decay half of its atoms. In this analogy, the cake represents the radioactive isotope, the act of eating represents the decay process, and the time it takes to eat half of the remaining cake each day mirrors the half-life, showing how both processes involve halving over consistent intervals.
Practice Version
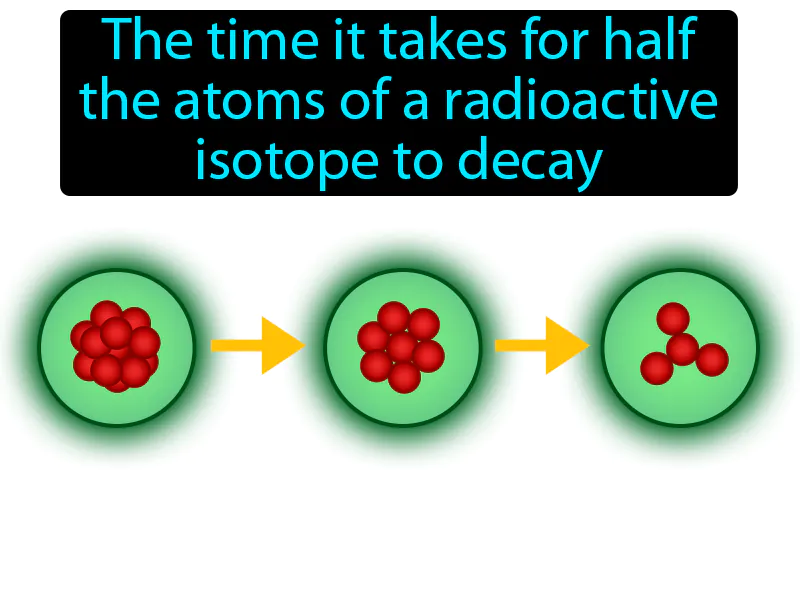
Half-life: The time it takes for half the atoms of a radioactive isotope to decay. Half-life. Half-life is the time it takes for half of the radioactive atoms in a sample to decay into something else.