Ionic Bond
What is an Ionic Bond? Explained in an easy to understand way:
What's Covered in the Video:
Imagine trying to fit the right puzzle pieces together, where only specific shapes can connect to complete the picture. This is similar to how ionic bonds work, where oppositely charged ions are like puzzle pieces that naturally attract and fit together due to their complementary charges. Just as a puzzle piece with a protruding tab needs a piece with a matching indentation to connect, a positively charged ion (like a sodium ion) is drawn to a negatively charged ion (like a chloride ion), forming a stable ionic bond as they come together to "complete the picture."
Practice Version
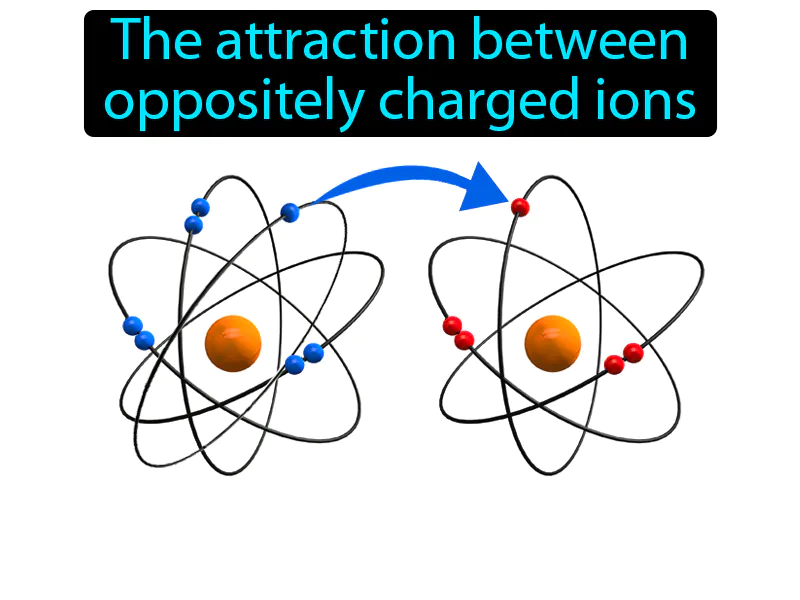
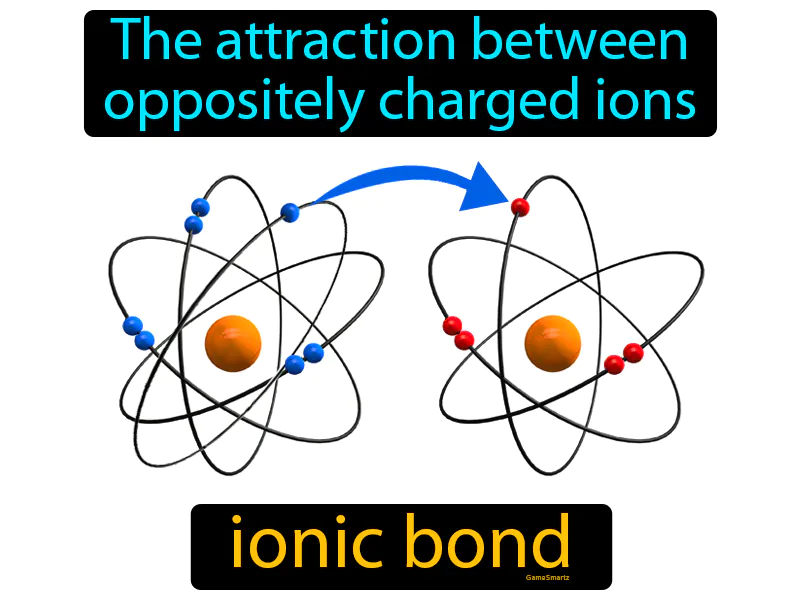
Ionic Bond: The attraction between oppositely charged ions. Ionic bond. An ionic bond is a strong connection formed when one atom donates an electron to another atom, resulting in positively and negatively charged ions that stick together.